Activating mutations in the BRAF gene, which are generally mutually exclusive from EGFR mutations or ALK rearrangements, act as an alternative oncogenic driver in metastatic non-small cell lung cancer (NSCLC). The most common of these mutations, BRAFV600E, is observed in 1–2% of lung adenocarcinomas. Combined BRAF and MEK inhibition has shown superior efficacy compared with BRAF inhibitor monotherapy in patients with BRAF-mutated metastatic melanoma, potentially contributing to sustained pathway inhibition and delay or prevention of resistance.
Three-fourths of patients with previously untreated BRAF V600E–mutated NSCLC, receiving a combination of a BRAF inhibitor dabrafenib plus a MEK inhibitor trametinib, achieved complete or partial response or stable disease by investigator assessment and independent review, according to findings from a phase II trial presented at ESMO 2017, the Annual Congress of the European Society for Medical Oncology in Madrid, Spain.
David Planchard, Department of Medical Oncology, Institute Gustave Roussy in Villejuif, France presented the results of the third (cohort C) of three sequentially enrolled cohorts in the phase II study of patients with BRAF V600E–mutated metastatic NSCLC (NCT01336634). In this cohort, 36 patients with BRAF V600E–mutated metastatic NSCLC received first-line treatment with 150 mg twice daily of dabrafenib and 2 mg once daily of trametinib. The patients had not received prior systemic therapy for metastatic disease.
Dr. Planchard pointed out that substantial clinical activity has been previously demonstrated by the combination in patients with previously treated BRAF V600E–mutated metastatic NSCLC (cohort B), who showed an investigator-assessed confirmed overall response rate (ORR) of 67%, median progression-free survival (PFS) of 10.2 months, and median overall survival (OS) of 18.2 months.
In the findings presented at ESMO 2017, the patients had a median age of 67 (range 44 to 91) years, 61% were female (61%), 83% were white, and 72% of patients were current or former smokers.
Investigator-assessed ORR was the primary endpoint and secondary endpoints included duration of response (DOR), PFS, overall OS, and safety.
Durable responses observed with dabrafenib and trametinib
At data cut-off on 8 April 2017, 11 (31%) patients remained on treatment.
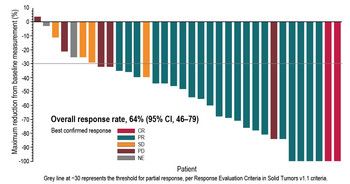
After a median follow-up of 15.9 months, the investigator assessed ORR was 64% (95% confidence interval [CI] 46%, 79%). Two (6%) patients receiving the combination experienced a complete response (CR), and 21 (58%) patients demonstrated partial response (PR). Overall, 4 (11%) patients had stable disease (SD) lasting ≥ 12 weeks as their best response, thus the disease control rate (DCR = CR+PR+SD) was 75% (95% CI 58%, 88%).
The independent review committee assessment supported these results.
The investigator-assessed median DOR was 10.4 (95% CI 8.3, 17.9) months. The median PFS was 10.9 (95% CI 7.0,16.6) months and median OS was 24.6 (95% CI 12.3, not estimable) months.
All (100%) of the patients experienced ≥ 1 adverse event (AE), and 69% had ≥ 1 grade 3/4 AE. Serious AEs (SAEs) occurring in > 2 patients included alanine aminotransferase increase in 14%, pyrexia in 11%, aspartate aminotransferase increase in 8%, and ejection fraction decrease was reported in 8% of patients.
A total of 24 patients progressed or died; of these 17 patients died. One patient death was due to due to a SAE of cardiorespiratory arrest that was determined to be unrelated to study treatment.
Regulatory agencies approvals
The US Food and Drug Administration (FDA) first approved the combination of dabrafenib and trametinib for patients with metastatic melanoma in January 2014. The European Commission approved the dabrafenib/trametinib combination for adults with unresectable or metastatic melanoma with a BRAF V600 mutation a year later.
In April 2017, the European Commission approved the combination of dabrafenib and trametinib for patients with BRAF V600-positive advanced or metastatic NSCLC.
On 22 June, 2017, the US FDA granted regular approvals to dabrafenib and trametinib administered in combination for patients with metastatic NSCLC with BRAF V600E mutation as detected by an FDA-approved test.
Conclusions
The investigators concluded that combined dabrafenib and trametinib represents a new targeted therapy with clinically meaningful antitumour activity and a manageable safety profile in patients with previously untreated BRAF V600E–mutated metastatic NSCLC, and noted that these results supported the recent approvals by the European Commission and US FDA.

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου