An investigation of immune cell subtypes in the tumour microenvironment of patients with lung adenocarcinoma and squamous cell carcinoma (SCC) identified an immune subgroup that associates with prolonged patient survival and may be prognostic of response to nivolumab, according to findings presented on 6 May, 2017 at the European Lung Cancer Conference (ELCC) in Geneva, Switzerland.
Lead author Dr Giulia Mazzaschi, Department of Medicine, University Hospital of Parma, in Parma, Italy and colleagues conducted this study to determine the putative impact of immunologically defined classes of non-small cell lung cancer (NSCLC) on clinical outcome following immunotherapy. Her research team investigated histologic sections of NSCLC samples surgically obtained from an untreated cohort of 51 patients with adenocarcinoma and 69 patients with SCC histology and sections from 8 patients with adenocarcinoma and 10 patients with SCC recruited to receive nivolumab.
Immunoperoxidase (H-score) and immunofluorescence were used to determine PD-L1 (clones 28-8, 22C3 and SP142) levels. The n/mm2 and intra-, peri-tumour, and invasive margin localisation of tumour infiltrating lymphocytes (TILs) subpopulations were computed to establish cut off values for each phenotype.
Improved survival seen in patients with type III NSCLC immune category
Kaplan Meier analysis of the immunohistochemical data and clinical records demonstrated that adenocarcinoma cases were 2-fold higher in CD3pos and 1.8-fold lower in CD4pos cells compared to SCC samples. Patients with adenocarcinoma having a higher population of TILs in the microenvironment experienced a 10-month increase in overall survival (OS) compared to patients with low levels of TILs (p < 0.01).
The investigators determined the intratumoral density of TILs to be lower in specimens harbouring an EGFR mutation.
The frequency of type I (PD-L1high TILshigh) contexture was low and was observed in just 14.6% of samples in this series. Type II (PD-L1low/neg TILslow) was detected in more than a third of NSCLC samples, reflecting immune exhaustion. In this series, a similar proportion of type III (PD-L1high TILslow), with increased natural killer and granzyme Bposcytotoxic cells, and IV (PD-L1low/neg TILshigh) enriched in T-regulatory cells, immune categories was observed.
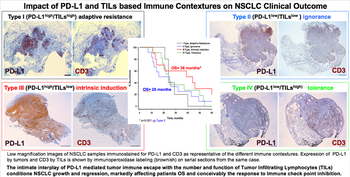
Impact of PD-L1 and TILs based immune contextures on NSCLC clinical outcome.
Credit: Dr Giulia Mazzaschi
Credit: Dr Giulia Mazzaschi
The NSCLC type III specimens showed a relationship with improved survival; patients with this type experienced median OS of 36.5 months and progression-free survival (PFS) of 27.6 months compared to OS of 25.7 months and PFS of 16.1 months in patients with type II specimens that were immune therapy naive. Patients having PD-1low levels plus a high CD8/CD3 ratio showed prolonged OS of 11 months compared to patients having PD-1high levels together with low CD8/CD3 ratios (p < 0.01). The subtype of patients with PD-L1high and PD-1low suggested a favourable response to immunotherapy, with 86% of these patients responding to nivolumab.
Conclusions
The authors conducted tissue characterisation of the immune contexture and determined which subgroups associated with improved OS and response to nivolumab immunotherapy. Based on the findings, they suggest that, in a dynamic PD-L1 milieu, a concomitant intrinsic or therapeutically induced decay of PD-1 receptor allows TILs to escape from PD-L1 pressure and delays tumour progression, thus improving OS.
Discussant point: Luis Paz-Ares from the Hospital Universitario Doce de Octubre, Madrid, Spain discussed the study findings in the context of impact of PD-L1 expression on clinical outcome, heterogeneous and complex immune milieu of NSCLC, NSCLC immune contextures and clinical outcome, impact of TILs on clinical outcome as the most robust prognostic marker among different studies has shown to be presence of TILs, subpopulations of TILs and clinical outcome, how can we predict CD8 T-cell reactivity against cancer antigens, and how PD-1/PD-L1 inhibitors response depends on CD28 signalling.

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου